An Experimental Drug For Ebola Is Now Getting A Second Shot Against COVID-19 In Malaysia
The World Health Organisation has chosen Malaysia as one of the countries to conduct joint research on the experimental antiviral compound called remdesivir as one of the four most promising therapies.
As cases of the new coronavirus disease continue to rise steeply around the globe, the World Health Organisation has accelerated its race to find possible treatments for the virus that causes COVID-19
On Friday, 27 March, a large global trial of four most promising coronavirus treatments called SOLIDARITY was announced by WHO to find out whether any can treat the COVID-19 infections.
According to a link shared by Health director-general Dr Noor Hisham Abdullah, "it's an unprecedented effort" that involves a "coordinated push to collect robust scientific data rapidly during a pandemic".
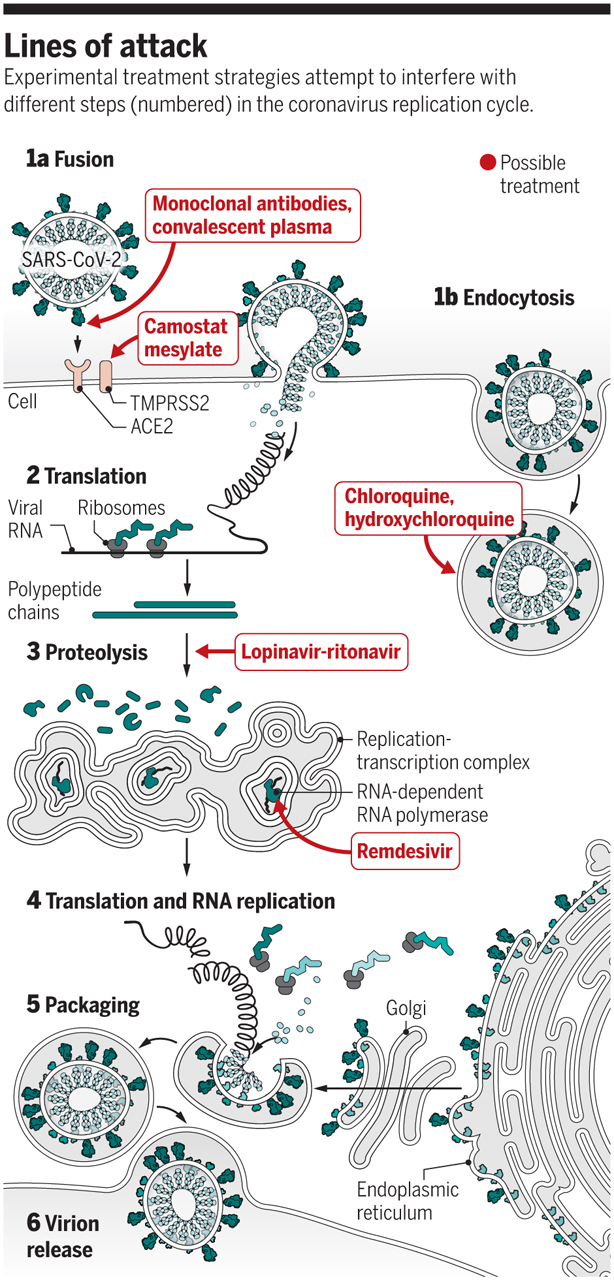
The four most promising therapies that WHO has identified are:
- Remdesivir, an experimental antiviral compound
- Chloroquine and hydroxychloroquine, malaria medications
- Ritonavir/lopinavir, a combination of two HIV drugs called Kaletra
- Ritonavir/lopinavir and interferon-beta, an immune system messenger that can help cripple viruses.
While the HIV combination, which has already been trialled in China and Malaysia among other countries, failed in a small study, WHO believes a large trial with a greater variety of patients is warranted.
How is this global trial of SOLIDARITY going to work?
As first noted by the well-known scientific journal called Science Magazine on 22 March, SOLIDARITY has made the enrollment of COVID-19 subjects for the trial easy.
An eligible case of COVID-19 will have their data along with underlying conditions that could change the course of the disease, such as diabetes or HIV infection, entered into a WHO website.
The participant will have to sign an informed consent form to be sent to WHO, which will then randomise the patient to one of the drugs that's available at the hospital or to the local standard care for COVID-19.
From this point on, physicians will record the day the patient left the hospital or died, the duration of the hospital stay, and whether the patient required oxygen or ventilation.
Among the four treatments in the trial, WHO has chosen Malaysia as one of the countries to conduct joint research on remdesivir
It's an antiviral, intravenous medicine that's been around for years as an experimental compound.
According to NPR, it's made by Gilead Sciences and has never been approved by America's the Food and Drug Administration — "or any other country's drug approval agency".
Remdesivir was initially trialled as a possible treatment against Ebola.
However, the experimental compound faltered, producing less impressive survival benefits against two other treatments that dramatically reduced deaths from the Ebola infection, reported STAT News.
On remdesivir's chances against COVID-19, Gilead's vice president of virology Tomas Cihlar said, "Drug discovery and development is usually a very long and tedious process and you could have many failures on the path to an approved product. It would be wonderful if it works. But it needs to be proven."
While clinical trials for remdesivir as a possible treatment against COVID-19 began in China in early February, NPR said that it's too soon to see final study results.
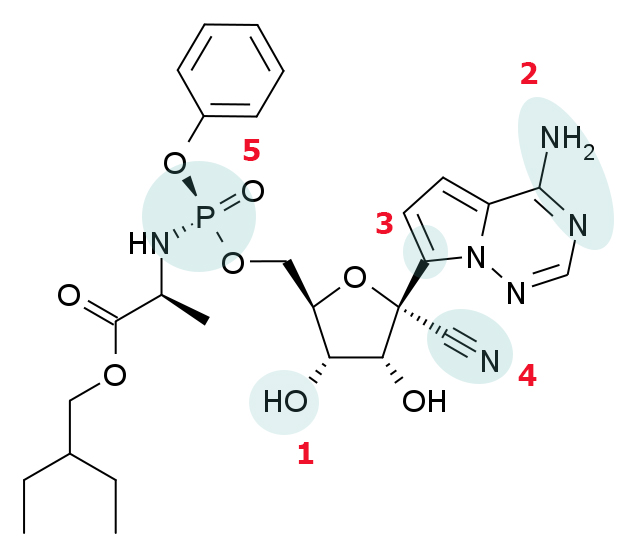
The chemical structure of remdesivir.
Image via American Society for Biochemistry and Molecular BiologyThe experimental drug, however, is getting a second shot in Malaysia because of evidence from individual cases that received treatment
A young man in Snohomish County in Washington, who was the first COVID-19 patient diagnosed in the US, was given remdesivir when his condition worsened. His condition improved the next day.
A patient in California, who doctors thought might not survive, recovered after he was given the same drug.
According to Jiang Shibo, a professor of virology at the School of Basic Medical Sciences, Fudan University in Shanghai, from the drugs in the trial "remdesivir has the best potential to be used in clinics".
Jiang, who has long worked on coronavirus therapeutics, cautioned that while we must urgently develop measures to tackle COVID-19, drugs shouldn't be deployed without sufficient safety guarantees.
"Testing vaccines and medicines without taking the time to fully understand safety risks could bring unwarranted setbacks during the current pandemic, and into the future," he said.
According to him, people's willingness to back quarantines and other public-health measures to slow spread tends to correlate with how much they trust the government's health advice.
"A rush into potentially risky vaccines and therapies will betray that trust and discourage work to develop better assessments. Despite the genuine need for urgency, the old saying holds: measure twice, cut once."
Malaysia was picked by WHO because of its ability to conduct the research and for the availability of good medical research facilities
A Malay Mail report said that we have the platform for the research to be conducted, which is Clinical Research Malaysia, adding that our researchers are also well trained to conduct such trial.
"So, among the reasons we were chosen is because we have been identified as a country with a good healthcare research system," Dr Noor Hisham was quoted as saying by The Edge Malaysia.
The research for the drug will take place at Sungai Buloh Hospital as well as those hospitals that have been identified as COVID-19 hospitals
The research will start once remdesivir is made available to the Ministry of Health by WHO.
Dr Noor Hisham said the antiviral drug will be given to COVID-19 patients and their conditions will be monitored for any side effects and also the effectiveness.
"The drug has been identified by WHO. Whether the drug is scientifically effective or not, we should first conduct the research," he was quoted as saying by New Straits Times on Friday.