MOH Bans These 9 Cosmetic Products Said To Contain Harmful Poisons
One of the brands also appears credible as they claim their products have been certified by the MOH themselves.
In a statement released yesterday, 15 July, the Ministry of Health (MOH) announced that they had banned the sale of nine cosmetic products said to contain harmful poisons
Health director-general (D-G) Datuk Dr Noor Hisham Abdullah noted that mercury and hydroquinone were found in the items.
The products are:
- Deeja Cosmetics Laika Cream: Contains mercury.- Deeja Cosmetics Zulai Cream: Contains hydroquinone.
- Deeja Cosmetic Yus Cream: Contains hydroquinone.
- Dnars Luvee Cream (Normal and Sensitive): Contains hydroquinone.
- Dnars Gold Lifthing Serum (Night): Contains mercury.
- Sparkle Beauty Cream: Contains hydroquinone, tretinoin, and betamethasone 17-valerate.
- Sparkle Sun Day Cream: Contains mercury.
- Qeziger Age-locking Recharging Spot A.M Cream: Contains mercury.
- MS Skinz Night Cream: Contains mercury.
According to the Health D-G, mercury can cause severe side-effects, including skin irritation, kidney damage, and disruptions to brain development in children
Additionally, mercury can negatively impact one's nervous system.
The statement continued, "Hydroquinone can cause redness to applied areas, discomfort, unintended changes to skin colour, increased hypersensitivity, hinder the process of pigmentation which protects from harmful UV rays, and increase the risk of skin cancer."
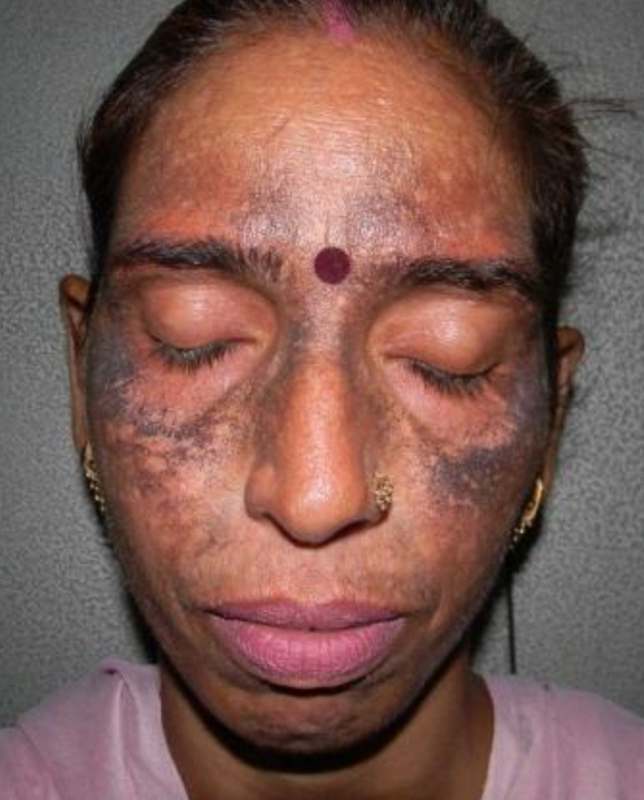
One of the side effects of hydroquinone is ochronosis, a blue-black discolouration to the skin.
Image via Egyptian Dermatology Online Journal (EDOJ)The poisons tretinoin and betamethasone 17-valerate, which were found in Sparkle Beauty Cream, should be registered under the Drug Control Authority and only used as prescribed by health professionals.
Dr Noor Hisham urged the public to immediately stop using the products and act according to the advice of trusted medical professionals
These products are being sold on e-commerce websites, such as Lazada and Shopee, for low prices.
Deeja Cosmetics also appear credible as it claims to be certified by the Ministry of Health themselves. On the other hand, Dnars Skincare suggests that their products are 100% free from mercury, tretinoin, and hydroquinone.
MS Skinz have since uploaded a statement to their Facebook page, suggesting that their product was sabotaged by those who bottled it
The post read, "The company has realised that a number of MS Skinz products have been replicated… Please get your products directly from the dealer to avoid being deceived."
The remaining companies have yet to comment on the matter.
Individuals who fail to comply with the prohibition will face up to RM25,000 in fines and three years in jail for the first offence
Dr Noor Hisham stressed that the sale and distribution of these products are a violation under the Control of Drugs and Cosmetics Regulations 1984.
Meanwhile, convicted companies will be fined up to RM50,000 for the first offence and up to RM100,000 for any subsequent violations.