Are Po Chai Pills Dangerous? Singapore's Health Authority Has Officially Cleared The Air
Some quick facts that will save you from all the panic.
You know that pill we grew up with that could basically cure 'everything'?
The reddish brown Po Chai pills are commonly found in Malaysian households as a traditional remedy to treat 'stomach problems', like indigestion, diarrhoea, bloating, vomiting and heartburn.
People would confidently say the pills can effectively cure just about everything, but at the same time draw a blank if you asked them what they're made of.
Wait, what are they made of? For the record, the pills are made of herbs and ingredients like roots, seeds, shoots, and leaves.
Po Chai pills are not to be mistaken for the Malaysian-made Teck Aun Chi Kit pills or fondly known as "Pil Chi Kit Teck Aun", that are also herbal pills for stomach aches.
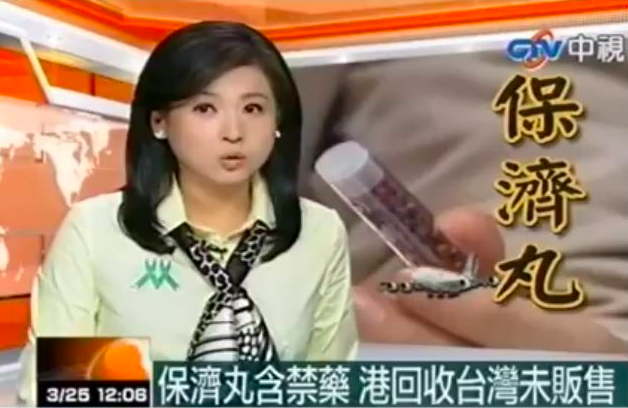
Recently, people were shocked to watch a video of a newscaster announcing that Po Chai pills contained cancer-causing ingredients
Imagine hearing that your favourite go-to medicine could give you cancer.
In that video, it was announced that a particular batch of Po Chai pills were taken off the shelves in Hong Kong after the pills were detected to have contained two dangerous ingredients: phenolphthalein (a banned laxative that has cancer-causing effects) and sibutramine, a banned western medicinal ingredient for slimming that can cause high blood pressure and serious cardiovascular effects.
The newscaster reveals that they were detected after tests were conducted by Singapore authorities. People, especially children and the elderly, were warned to stop taking the pills immediately and to consult a doctor if they felt ill after taking them.
Understandably so, this news spread across WhatsApp chat groups and Facebook, causing panic among Malaysian consumers who have been eating these 'miracle' pills since they could remember.
So, are Po Chai pills safe to eat? The Health Sciences Authority (HSA) of Singapore has cleared a few things up
1. First things first, that video is from 2010 so it's pretty outdated
Well, we could've figured that out ourselves with a quick Google search, but thank you, HSA!
The recall in Hong Kong happened seven years ago after Singaporean authorities detected in a routine check that two banned chemical substances were found in a batch of Po Chai capsules.
2. No harmful substances were found in Po Chai pills
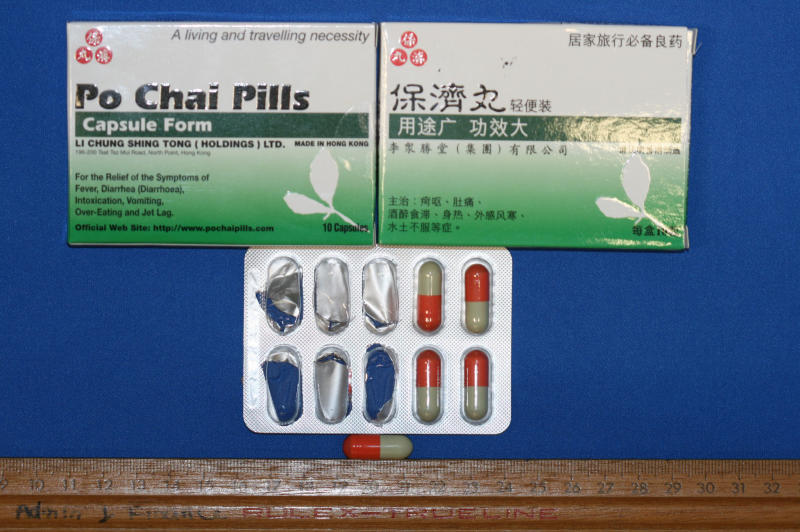
Po Chai products manufactured by Li Chung Shing Tong (Holdings) Limited in Hong Kong, used to be available in two forms - capsules and pills.
The two banned chemical substances were only found in their capsules, not pills.
"Following HSA's detection of the disallowed substances in Po Chai Pills (Capsule Form) in 2010, Hong Kong's Department of Health (DOH) recalled all the Po Chai Pills products. As Po Chai Pills (Bottle Form) was not affected, DOH subsequently allowed the manufacturer to resume production and marketing of the pills," read the official statement from Singapore's Health Sciences Authority (HSA).
3. The manufacturer has stopped producing the capsules since 2010
The manufacturer ceased production of the capsules in 2010 after the product was recalled. In a March 2010 report by South China Morning Post (SCMP), it was said that the Hong Kong based manufacturer, Li Chung Shing Tong, could face up to five charges for misconduct.
It was also exposed that although Po Chai capsules were already on sale, the manufacturer's application for their registration was actually still in process.
"... in its application, the company did not report that the capsule had been found to contain cancer-causing ingredients. This could constitute grounds for a charge of false declaration."
4. A lab test in June 2016 proved that the product was compliant with Singapore's quality standards
Po Chai pills are manufactured by Li Chung Shing Tong (Holdings) Limited in Hong Kong
Image via South China Morning PostThe official statement from HSA says, "The product did not contain phenolphthalein, sibutramine, or any other western potent medicinal ingredients or contaminants."
5. Po Chai pills are still being sold in Singapore and monitored as usual
HSA continues to monitor the product under their product quality surveillance programme.